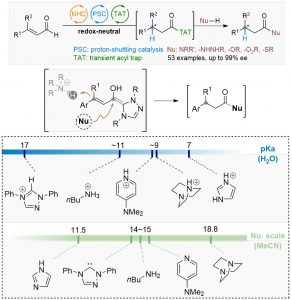
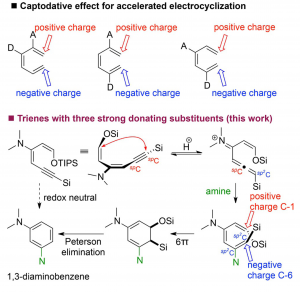
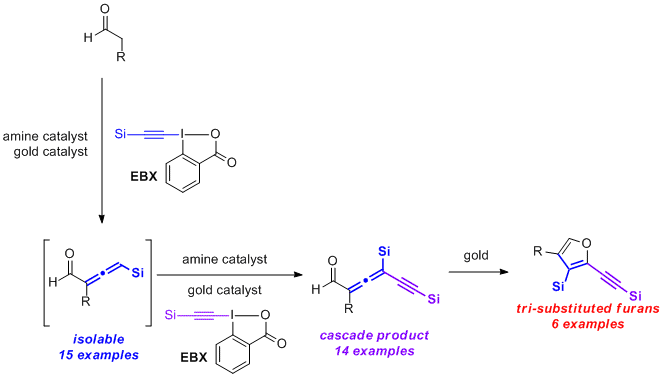
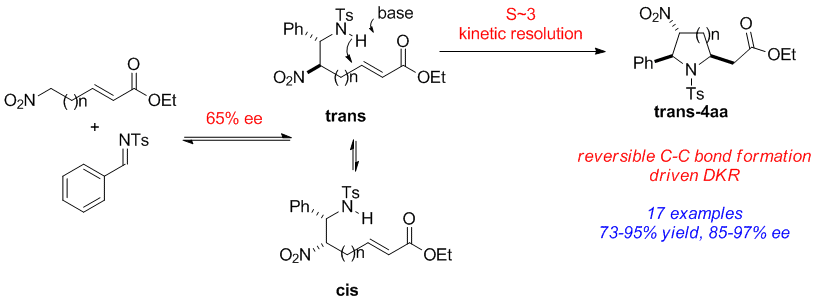
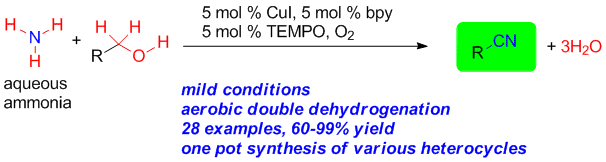
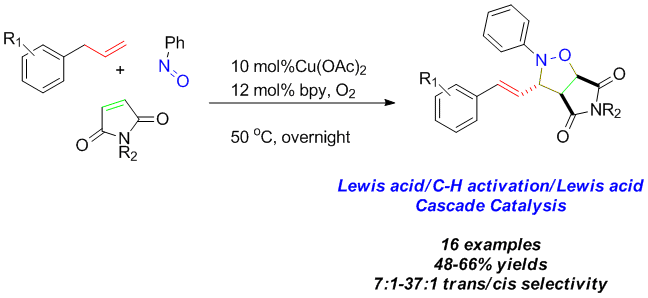
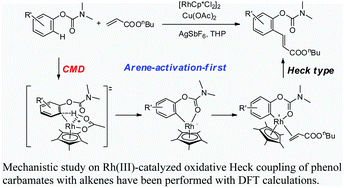
86. Water mediated redox-neutral cleavage of arylalkenes via photoredox catalysis
Ke Liao, Yuqi Fang, Lei Sheng, Jiean Chen,* Yong Huang*
Nat. Commun. 2024, 15, 6227.

Xingchen Ye, Huaijin Pan, Yong Huang,* Jiean Chen,* Zhaofeng Wang*
Chem. Sci. 2024, 15, 6515-6521.

Jing Wang, Xinyang Zhao, Yucheng Tao, Xiuxiu Wang, Li Yan, Kuang Yu, Yi Hsu, Yuncong Chen, Jing Zhao,* Yong Huang,* Wei Wei*
Nat. Commun. 2024, 15, 3534.

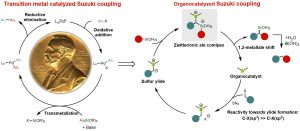
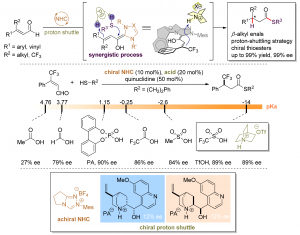
83. Amide C–N bonds activation by A new variant of bifunctional N-heterocyclic carbene
Yuxing Cai, Yuxin Zhao, Kai Tang, Hong Zhang, Xueling Mo, Jiean Chen,* Yong Huang*
Nat. Commun. 2024, 15, 496.
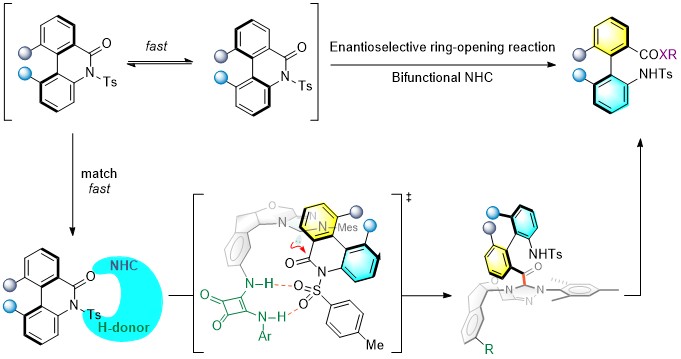
82. Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides
Fengjin Wu, Yichi Zhang, Ruiqi Zhu, Yong Huang*
Nat. Chem. 2024, 16, 132-139.
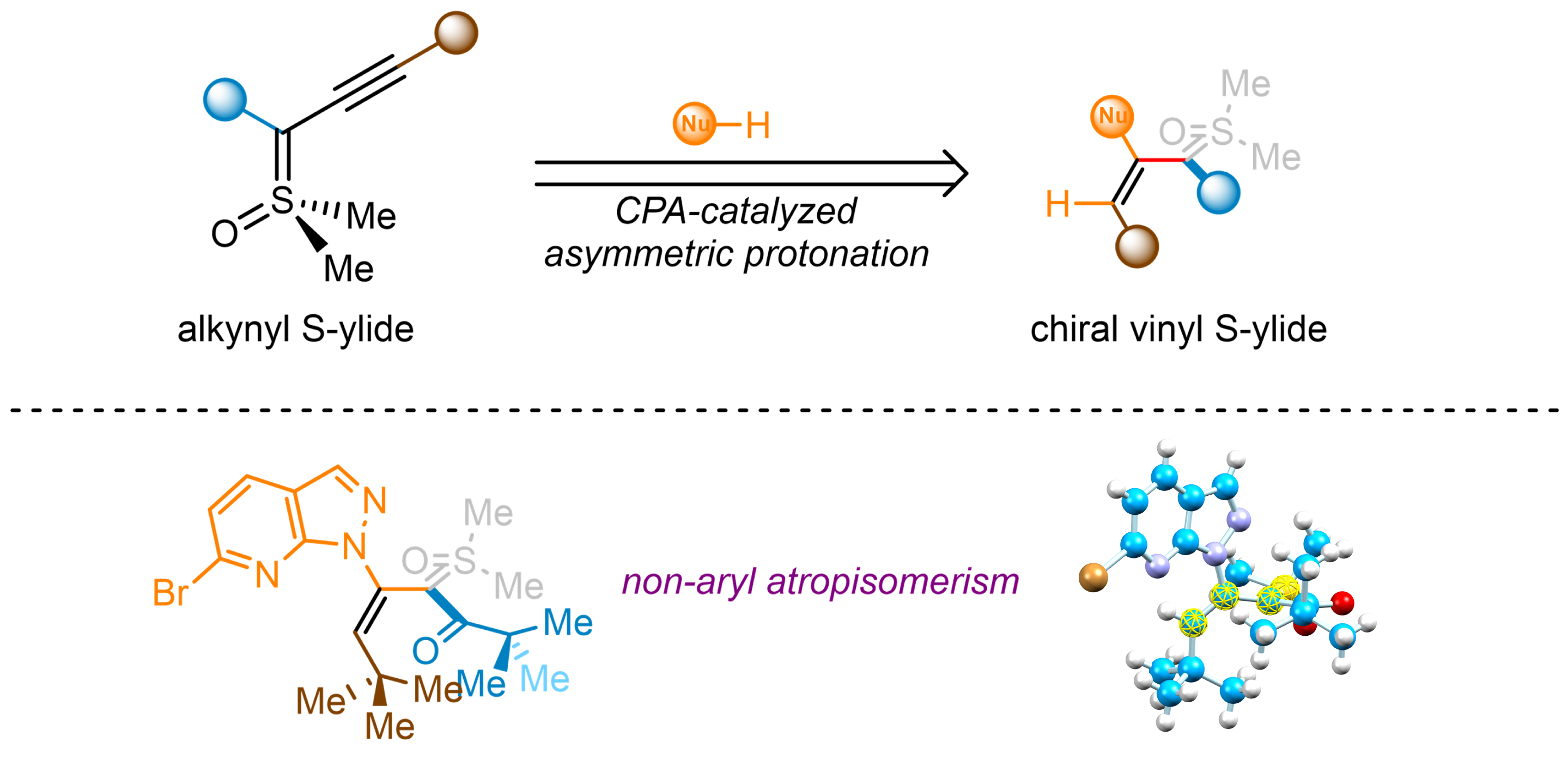
81. A Carbene Relay Strategy for Cascade Insertion Reactions
Li Li, Chenggang Mi, Guanwang Huang, Meirong Huang, Yuyi Zhu, Shao-Fei Ni, Zhaofeng Wang,* Yong Huang*
Angew. Chem. Int. Ed. 2023, e202312793

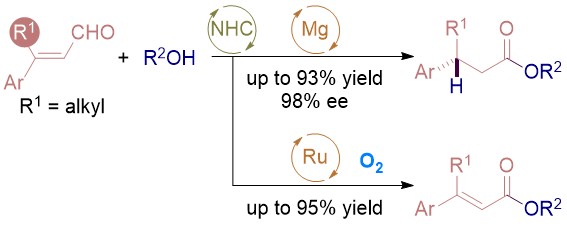
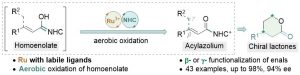
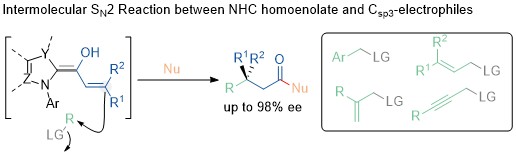
80. Enantioselective SN2 Alkylation of Homoenolates by N-Heterocyclic Carbene Catalysis
En Li, Kai Tang, Zhuhui Ren, Xiaoyun Liao, Qianchen Liu, Yong Huang,* Jiean Chen*
Adv. Sci. 2023, 2303517
Kalok Chan, Long Thanh Ta, Yong Huang,* Haibin Su,* Zhenyang Lin*
Molecules 2023, 28, 4730
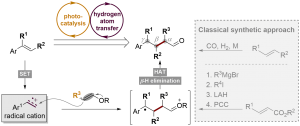
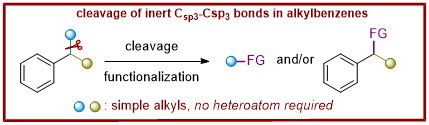
78. Photoredox Cleavage of a Csp3–Csp3 Bond in Aromatic Hydrocarbons
Ke Liao, Cho Ying Chan, Siqi Liu, Xinhao Zhang, Jiean Chen*, Yong Huang*
J. Am. Chem. Soc. 2023, 145, 12284–12292
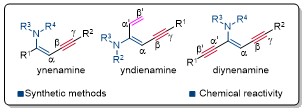
77. Recent Advances in Ynenamine Chemistry
BingyangHan, ZhaofengWang,* YongHuang*
Chem. Rec. 2023, 23, e202300099 (Invited Account)
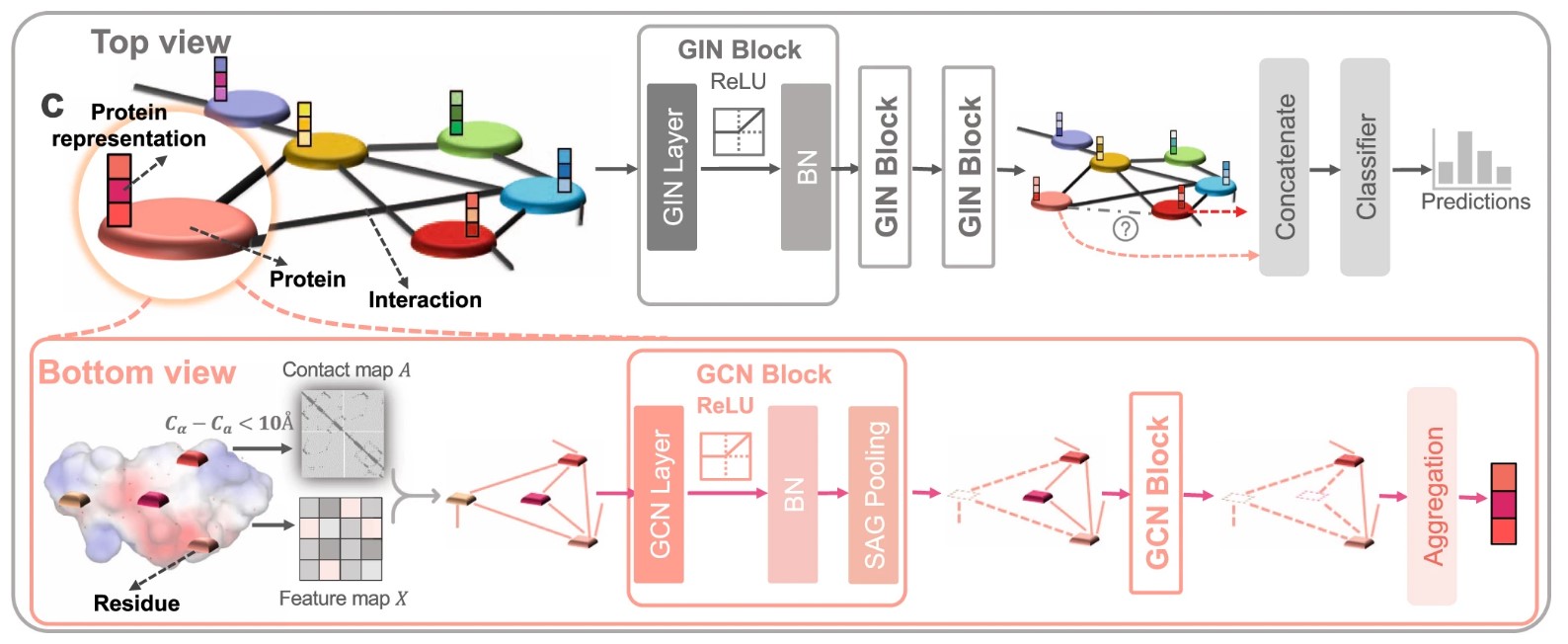
76. Hierarchical graph learning for protein–protein interaction
Ziqi Gao, Chenran Jiang, Jiawen Zhang, Xiaosen Jiang, Lanqing Li, Peilin Zhao, Huanming Yang, Yong Huang,* Jia Li*
Nat. Commun. 2023, 14, 1093.
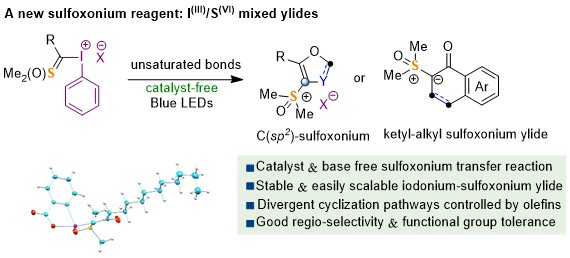
Li Li, Kun Deng, Yajie Xing, Cheng Ma, Shao-Fei Ni, Zhaofeng Wang,* Yong Huang* Nat. Commun. 2022, 13, 6588.
74. A Thioether-Catalyzed Cross-Coupling Reaction of Allyl Halides and Arylboronic Acids
Jingwei Xu, Zhiqi He, Jiwei Zhang, Jiean Chen,* Yong Huang* Angew. Chem. Int. Ed. 2022, 61, e202211408.
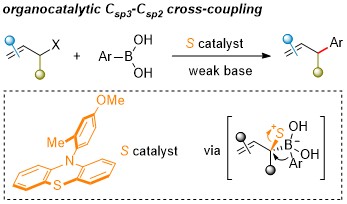
73. An NHC-catalyzed [3+2] cyclization of β-disubstituted enals with benzoyl cyanides
Wangsheng Liu, Linrui Zhang, Xiaoyun Liao, Jiean Chen,* Yong Huang* Chem. Commun. 2022, 58, 9742-9745.
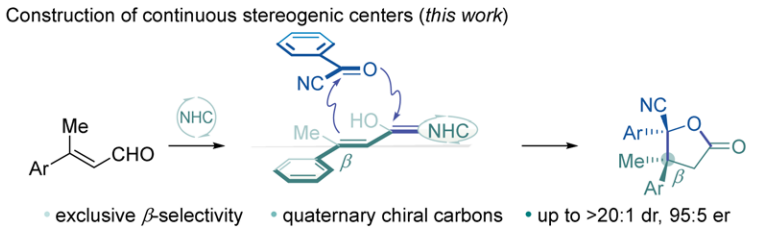

Ke Liao, Fengjin Wu, Jiean Chen,* Yong Huang* Cell Rep. Phys. Sci. 2022, 3, 100763.
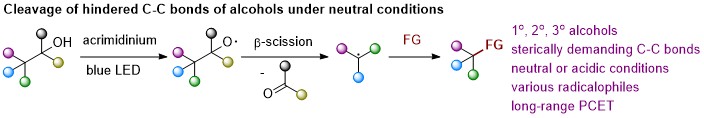
En Li, Jiean Chen,* Yong Huang* Angew. Chem. Int. Ed. 2022, 61, e202202040.
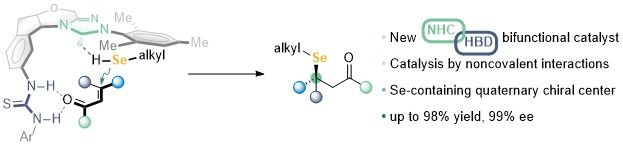
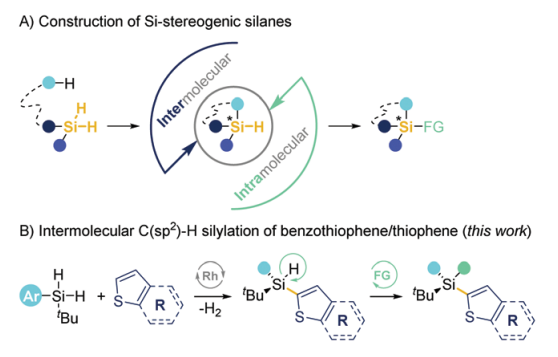
70. Enantioselective synthesis of acyclic monohydrosilanes by steric hindrance assisted C-H silylation
Delong Mu, Shuqiong Pan, Xiaoyu Wang, Xiaoyun Liao, Yong Huang*, Jiean Chen* Chem. Commun. 2022, 58, 7388-7391.
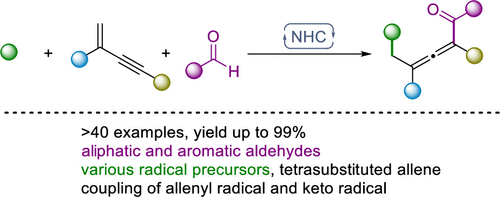
69. N-Heterocyclic Carbene-Catalyzed 1,4-Alkylcylation of 1,3-Enynes
Yuxing Cai, Jiean Chen,* and Yong Huang.* Org. Lett. 2021, 23, 9251-9255.
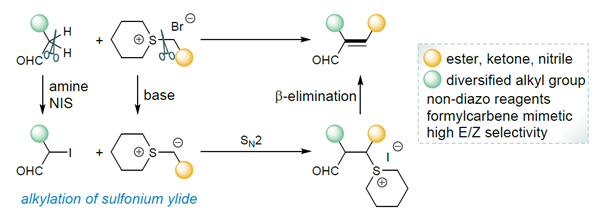
68. A cross-coupling Reaction between Aliphatic Aldehydes and Sulfonium Salts
Baoli Chen, Li Li, Jiean Chen,* and Yong Huang.* Adv. Synth. Catal. 2022, 364, 30-34.
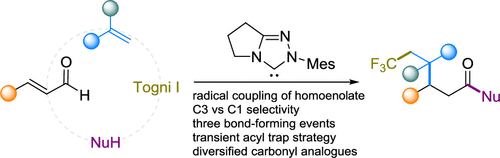
Zhen Li, Meirong Huang, Xinhao Zhang, Jiean Chen,* and Yong Huang.* ACS catal. 2021, 11, 10123-10130.
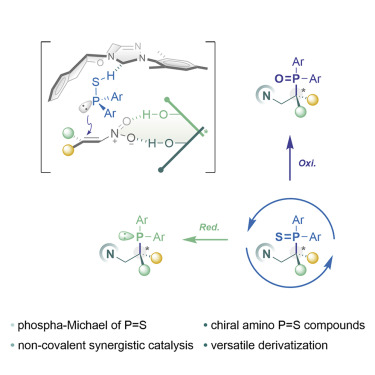
66. Synthesis of a-chiral phosphine sufides via no-covalent organocatalysis
En Li, Qian Wang, Yuxing Cai, Jiean Chen,* and Yong Huang.* Cell Reports Physical Science. doi:rg/10.1016/j.xcrp.2021.10.
Fangfang Guo, Jiean Chen,* and Yong Huang.* ACS catal. 2021, 11, 6316-6324.
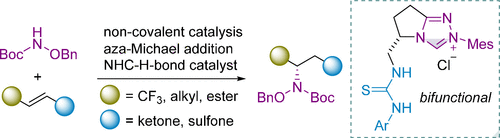
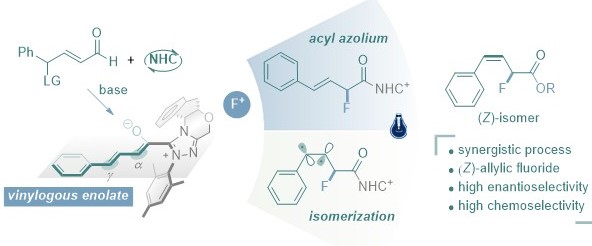
Xinhang Jiang, En Li, Jiean Chen,* and Yong Huang.* Chem. Commun., 2021, 57, 729-732.
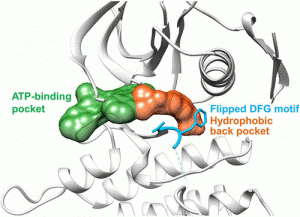
63. MolecularInsightsintoSmall-MoleculeDrugDiscovery forSARS-CoV-2
Hailei Su, Feng Zhou, Ziru Huang, Xiaohua Ma, Kathiresan Natarajan, Minchuan Zhang, Yong Huang,* and Haibin Su.* Angew. Chem. Int. Ed. 2021, 60, 9789–9802.

62. Enantioselective Intramolecular [2,3]-Sigmatropic Rearrangement of Aldehydes via a Sulfonium Enamine Intermediate
Li Li, Baoli Chen, Jiean Chen,* Yong Huang.* Angew. Chem. Int. Ed. 2020, 59, 20904 –20908.

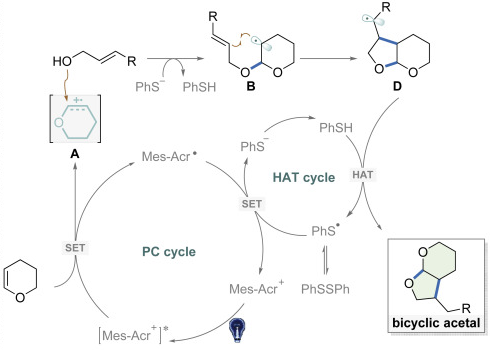
61. Direct Synthesis of Bicyclic Acetals via Visible Light Catalysis
Fengjin Wu, Leifeng Wang, Ying Ji, Ge ou, Hong Shen, David A. Nicewicz,* Jiean Chen,* Yong Huang.* iScience 2020, 23, 10139.
Li Li, Feifei Song, Xiumei Zhong, Yun-Dong Wu, Xinhao Zhang,* Jiean Chen,* and Yong Huang.* Adv. Synth. Catal. 2020, 362, 126 – 132
59. Alcohol-Directed ortho-C–H Alkenylation
Li Li, Qinglan Liu, Jiean Chen *, Yong Huang *. Synlett 2019, 30, A-E. DOI: 10.1055/s-0037-1611538
58. Enantio- and Diastereoselective Hydrofluorination of Enals via N-Heterocyclic Carbene Catalysis
Leming Wang, Xinhang Jiang, Jiean Chen,* Yong Huang*. Angew. Chem. Int. Ed. 2019. DOI:10.1002/anie.201902989
57. N‐Heterocyclic Carbenes as Brønsted Base Catalysts
Jiean Chen, Yong Huang. Book Author(s): Akkattu T. Biju. DOI :10.1002/9783527809042.ch9
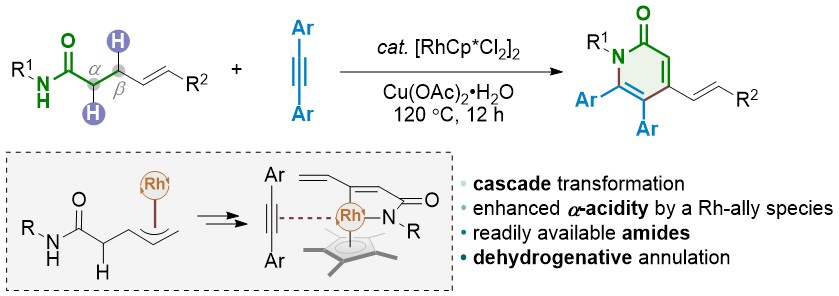
56. Dehydrogenative Annulation of γ,δ-Unsaturated Amides and Alkynes via Double C-H Activation
Wang Zhen, Li En, He Zhiqi, Chen Jiean, Huang Yong. Acta Phys. -Chim. Sin. 2019, 35, 0001–0009. DOI: 10.3866/PKU.WHXB201811038.
55.Switching Reaction Pathways by Cooperative Catalysis of N-Heterocyclic Carbene and Lewis Acids
Wang Leming, Wang Qian, Chen Jiean*, Huang Yong*. Acta Chim. Sinica 2018, 76, 850—856.
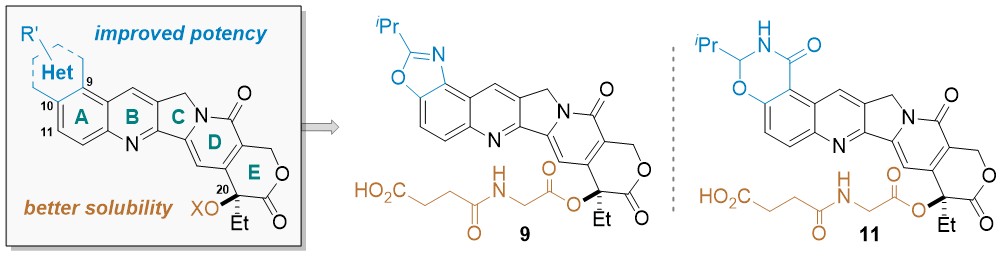
54. Structure-Based Drug Design and Identification of H2O‑Soluble and Low Toxic Hexacyclic Camptothecin Derivatives with Improved Efficacy in Cancer and Lethal Inflammation Models in Vivo
Peichen Pan,† Jiean Chen,§ Xijian Li,§ Miyang Li,§ Huidong Yu,∥ Jean J. Zhao,⊥,# Jing Ni,⊥,#Xuwen Wang,† Huiyong Sun,† Sheng Tian,∇ Feng Zhu,† Feng Liu,∇ Yong Huang,*,§ and Tingjun Hou*,†,‡ J. Med. Chem. 2018, XXX, XXX−XXX. DOI: 10.1021/acs.jmedchem.8b00498
53. Aerobic Oxidation/Annulation Cascades via Synergistic Catalysis of RuCl3 and N‐Heterocyclic Carbenes
52. Enantioselective hydroamidation of enals by trapping of a transient acyl species
Pengfei Yuan, Jiean Chen*, Jing Zhao*, Yong Huang*. Angew. Chem. Int. Ed. 2018. 57, 8503 –8507. DOI: 10.1002/anie.201803556
Zhiqi He, Feifei Song, Huan Sun, and Yong Huang*. J. Am. Chem. Soc. 2018, 140, 2693-2699. DOI: 10.1021/jacs.8b00380
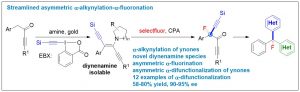
50. Streamlined asymmetric α-difunctionalization of ynones
Siyu Peng1, Zhaofeng Wang1, Linxing Zhang, Xinhao Zhang* & Yong Huang*. Nat. Commun. 2018, 9, 375. DOI: 10.1038/s41467-017-02801-9
49. Enantioselective Cooperative Proton-Transfer Catalysis using Chiral Ammonium Phosphates
Linrui Zhang, Pengfei Yuan, Jiean Chen* and Yong Huang*. Chem. Commun., 2018, 54, 1473. DOI: 10.1039/C7CC09549J
48. Direct Synthesis of Polysubstituted Aldehydes via Visible-Light-Catalysis
Fengjin Wu1, Leifeng Wang1, Jiean Chen*, David A. Nicewicz*, Yong Huang*. Angew. Chem. Int. Ed. 2018, 57, 2174-2178. DOI: 10.1002/anie.201712384
Zhen Wang1, Zhiqi He1, Linrui Zhang and Yong Huang*, J. Am. Chem. Soc., 2018, 140, 735−740. DOI: 10.1021/jacs.7b11351
46. Amine-Triggered 6p-Electrocyclization–Aromatization Cascade of Ynedienamines
Xijian Li, Huidong Yu, and Yong Huang*, Adv. Synth. Catal. 2017, 359, 1379 – 1387
Peichen Pan, Huidong Yu, Qinglan Liu, et al. Yong Huang*, and Tingjun Hou*, ACS Cent. Sci. 2017, DOI: 10.1021/acscentsci.7b00419
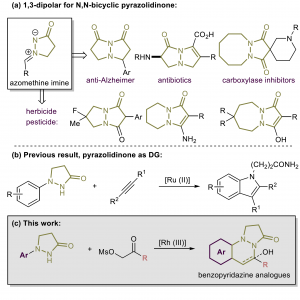
44. Construction of Pyridazine Analogues via Rhodium-mediated C-H Activation
Chao Yang, Feifei Song, Jiean Chen*, and Yong Huang*, Adv. Synth. Catal. 2017, 359, 3496-3502.
43. Enantioselective β‑Protonation of Enals via a Shuttling Strategy
Jiean Chen1, Pengfei Yuan1, Leming Wang, and Yong Huang*, J. Am. Chem. Soc. 2017, 139, 7045−7051.
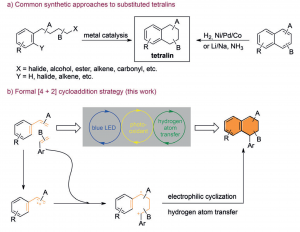
42. Visible Light Mediated [4+2] Cycloaddition of Styrenes Synthesis of Tetralin Derivatives
Leifeng Wang, Fengjin Wu, Jiean Chen, David A. Nicewicz,* and Yong Huang*, Angew. Chem. Int. Ed. 2017, 56, 6896-6900.
Fei Wang, Jiean Chen* and Yong Huang*, Synlett 2017,28, 1300-1304. (Invited Synlett Cluster)
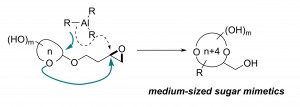
40. A Migratory Ether Formation Route to Medium-Sized Sugar Mimetics
Hao Jiang, Li-Ping Xu, Yan Fang, Zhen-Xing Zhang, Zhen Yang,* and Yong Huang*, Angew. Chem. Int. Ed., 2016, 55, 14340 –14344.
39. Diverting C−H Annulation Pathways Nickel-Catalyzed Dehydrogenative Homologation of Aromatic Amides
Zhiqi He and Yong Huang*, ACS Catal. 2016, 6, 7814−7823.
Huan Sun, Nicolas Guimond* and Yong Huang*, Org. Biomol. Chem., 2016,14, 8389–8397.(Invited Perspective)
37. Ligand-Assisted Palladium(II)/(IV) Oxidation for sp3 C-H Fluorination
Huan Sun, Yi Zhang, Ping Chen, Yun-Dong Wu*, Xinhao Zhang* and Yong Huang*, Adv. Synth. Catal., 2016, 358, 1946.(Very Important Paper, Front Cover)
36. New frontiers of N-heterocyclic carbene catalysis
Jiean Chen and Yong Huang*, Sci. China Chem., 2016, 59, 251(Invited Perspective)
Pengfei Yuan, Sixuan Meng, Jiean Chen* and Yong Huang*, Synlett, 2016, 27, 1068.(Invited Synlett Cluster)
Huan Sun and Yong Huang*, Synlett. 2015, 26, 2751. (Invited Account)
Leming Wang, Jiean Chen and Yong Huang*, Angew. Chem. Int. Ed. 2015, 54, 15414.
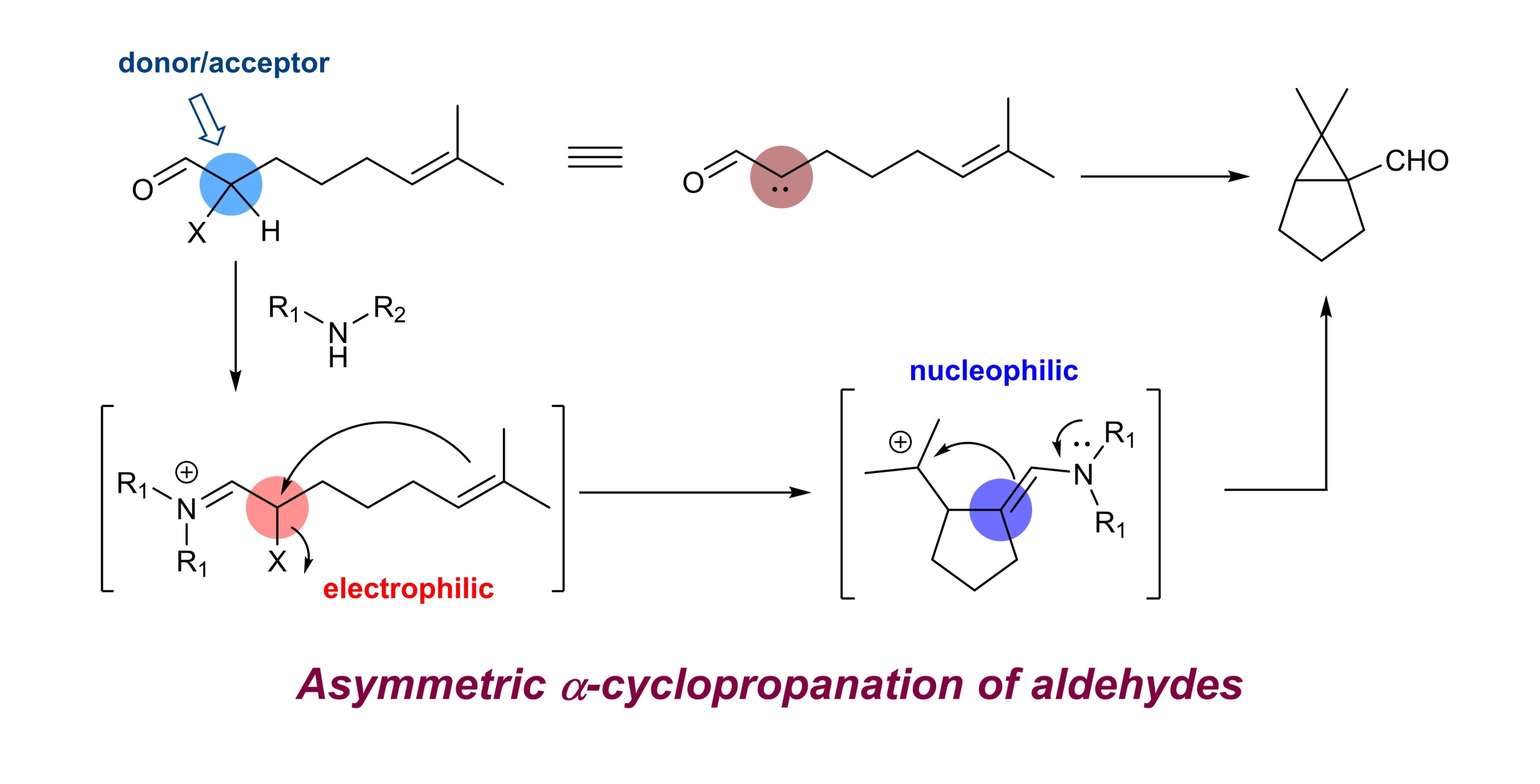
32. Asymmetric intramolecular α-cyclopropanation of aldehydes using a donor/acceptor carbene mimetic
Chaosheng Luo, Zhen Wang and Yong Huang*, Nature Commun. 2015, 6, 10041.
31. Highly enantioselective sulfa-Michael addition reactions using N-heterocyclic carbene as a noncovalent organocatalyst
Jiean Chen, Sixuan Meng, Leming Wang, Hongmei Tang and Yong Huang*, Chem. Sci. 2015, 6, 4184.
30. A Copper-Catalyzed Aerobic Domino Process for The Synthesis of Isoindolin-1-ylidene Derivatives
Hu Chen, Qian Wang and Yong Huang*, Tetrahedron 2015, 71, 3632. (Invited Contribution for a Special Issue)
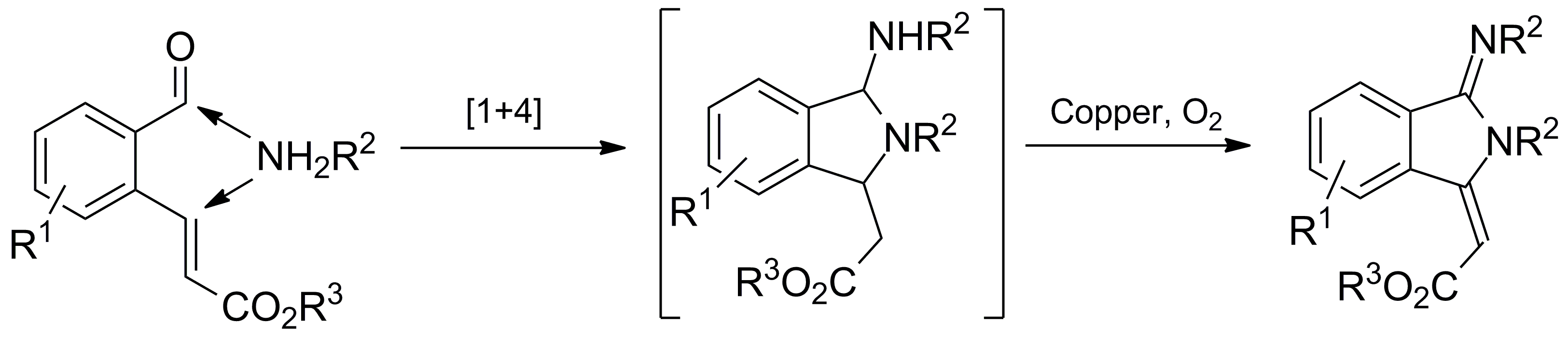
Xijian Li, Siyu Peng, Li Li and Yong Huang*, Nature Commun. 2015, 6, 6913.
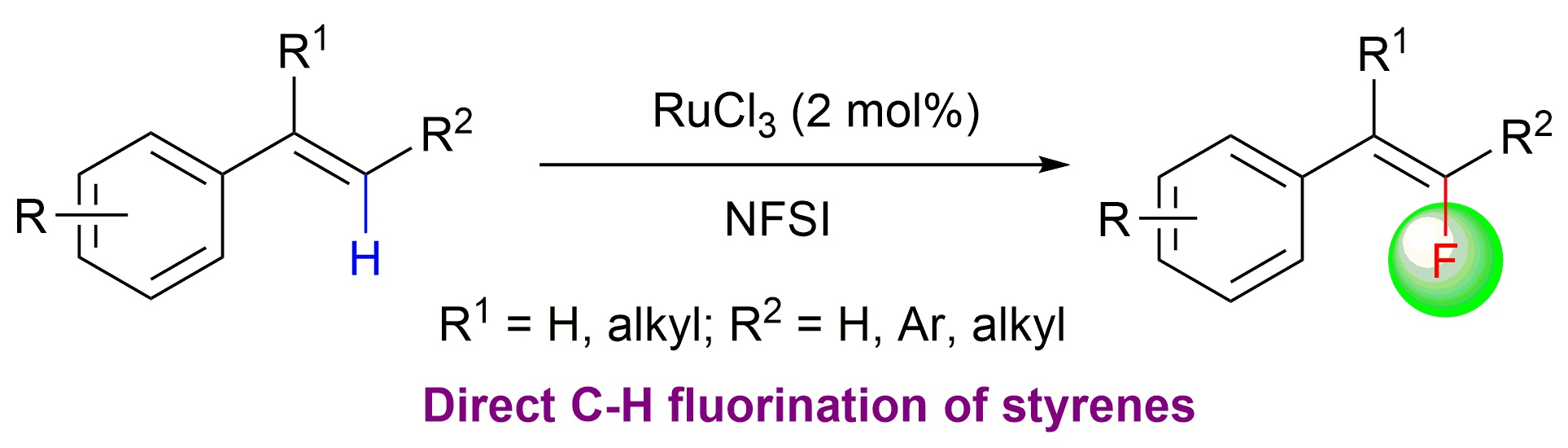
28. Direct Fluorination of Styrenes
Qian Shao, and Yong Huang*, Chem. Commun. 2015, 51, 6584-6586.
27. Synthesis of Indolo[2,1-a]isoquinolines via a Triazene-Directed C–H Annulation Cascade
Huan Sun, Chengming Wang, Yun-Fang Yang, Ping Chen, Yun-Dong Wu*, Xinhao Zhang* and Yong Huang*, J. Org. Chem. 2014, 79, 11863-11872.
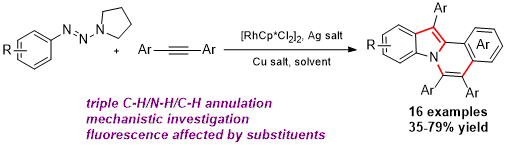
Zhenxing Zhang, Hao Jiang and Yong Huang*, Org. Lett. 2014, 16, 5976–5979.
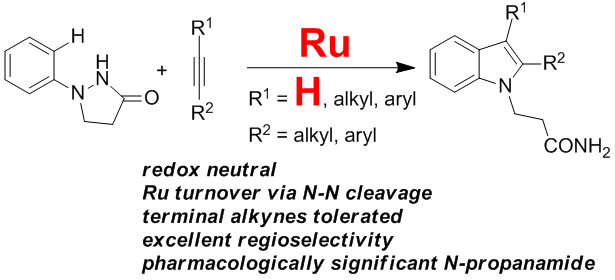
25. Directed Arenealkyne Annulation Reactions via Aerobic Copper Catalysis
Yi Zhang, Qian Wang, Huidong Yu* and Yong Huang*, Org. Biomol. Chem. 2014, 12, 8844-8850.
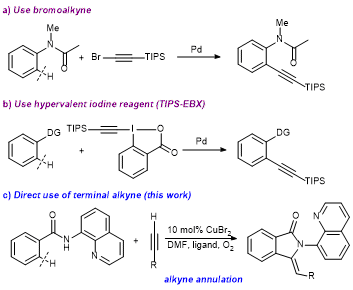
24. Highly ortho-Selective Trifluoromethylthiolation Reactions using a Ligand Exchange Strategy
Weiyu Yin, Zhaofeng Wang and Yong Huang*, Adv. Synth. Catal. 2014, 356, 2998 – 3006.
Zhaofeng Wang, Li Li and Yong Huang*, J. Am. Chem. Soc. 2014, 136, 12233–12236.
Ying Chen, Dongqi Wang, Pingping Duan, Rong Ben, Lu Dai, Xiaoru Shao, Mei Hong, Jing Zhao* and Yong Huang*, Nature Commun. 2014, 5, 4610.
21. Asymmetric catalysis with N-heterocyclic carbenes as non covalent chiral templates
Jiean Chen and Yong Huang*, Nature Commun. 2014, 5, 3437.
Yan Fang, Chengming Wang, Shengqin Su, Haizhu Yu* and Yong Huang*, Org. Biomol. Chem. 2014,12, 1061-1071.
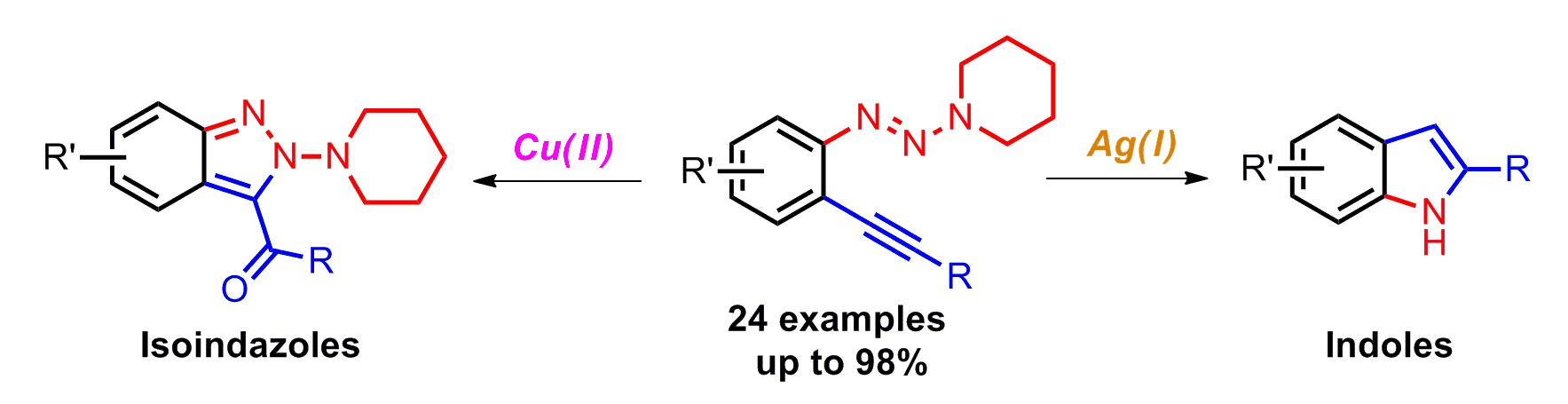

Tao Cheng, Weiyu Yin, Yi Zhang, Yingnan Zhang and Yong Huang*, Org. Biomol. Chem. 2014,12, 1405-1411.
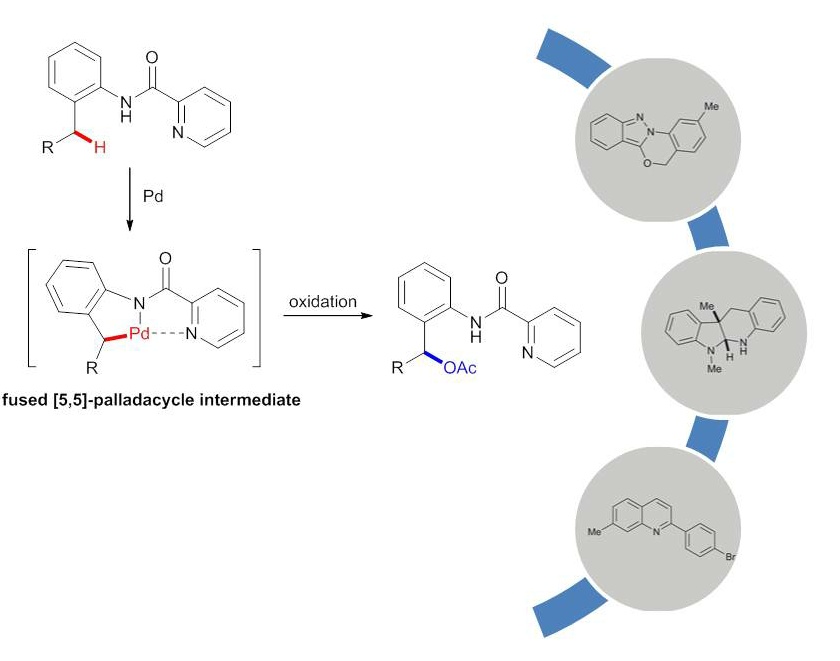
Zhaofeng Wang, Xijian Li, Yong Huang*, Angew. Chem. Int. Ed. 2013, 42, 14219-14223.
Junlin Zhang, Leming Wang, Qi Liu, Zhen Yang*, and Yong Huang*, Chem. Commun. 2013, 49, 11662-11664.
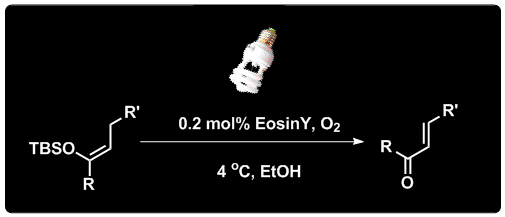
Qian Shao, Jiean Chen, Meihua Tu, David W. Piotrowski and Yong Huang*, Chem. Commun. 2013, 49, 11098-11100.
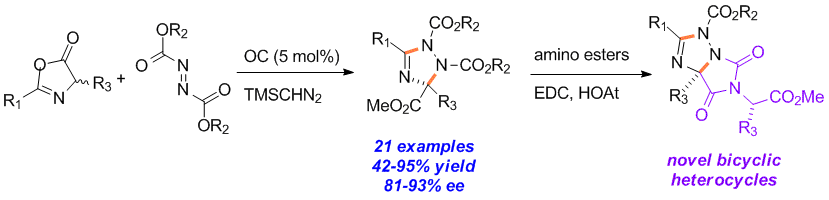
15. Traceless Directing Strategy Efficient Synthesis of N-Alkyl Indoles via Redox-Neutral C–H Activation
Chengming Wang and Yong Huang*, Org. Lett. 2013, 15, 5294-5297.
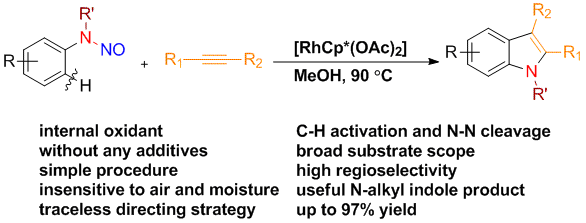
Chaosheng Luo and Yong Huang*, J. Am. Chem. Soc. 2013, 135, 8193–8196.
Highlighted by SYNFACTS, 2013, 9(8), 0898; DOI-10.1055s-0033-1339442.
13. General and Efficient Synthesis of Indoles through Triazene Directed C–H Annulation
Chengming Wang, Huan Sun, Yan Fang, and Yong Huang*, Angew. Chem. Int. Ed. 2013, 52, 5795-5798. (cover)
Highlighted by Chinese-Journal-of-Organic-Chemistry-2013-337-1593.


Tao Cheng, Sixuan Meng, and Yong Huang*, Org. Lett. 2013, 15 , 1958–1961.
Weiyu Yin, Chengming Wang and Yong Huang*, Org. Lett. 2013, 15, 1850–1853.
Highlighted-by-SYNFACTS-2013-97-0722.-DOI_-10.1055_s-0033-1339227., Highlighted by organic chemistry portal
Hu Chen, Zhaofeng Wang, Yingnan Zhang and Yong Huang*, J. Org. Chem. 2013, 78, 3503-3509.
Highlighted by SYNFACTS, 2013, 9(6), 0602. DOI: 10.1055/s-0033-1338792.
Qi Zhang, Hai-Zhu Yu, Yi-Tong Li, Lei Liu, Yong Huang* and Yao Fu*, Dalton Trans. 2013, 42, 4175-4184.
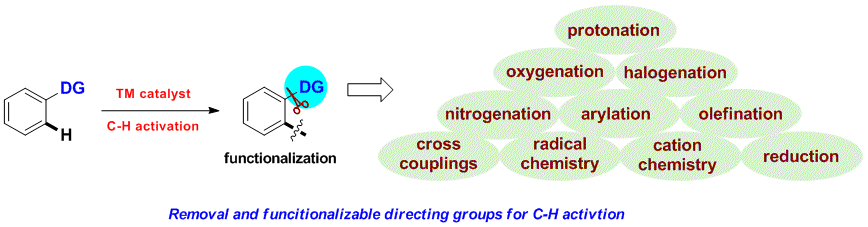
8. Expanding Structural Diversity Removable and Manipulable Directing Groups for C-H Activation
Chengming Wang, Yong Huang*, Synlett. 2013, 24,145.(invited)
Yong Huang, Weiming Gao, Torbjõrn Åkermark, Mingrun Li, BjÖrn Åkermark*, Eur. J. Inorg. Chem. 2012, 27, 4259-4263.
Chengming Wang, Hu Chen, Zhaofeng Wang, Jiean Chen and Yong Huang*, Angew. Chem. Int. Ed. 2012, 51, 7242-7245. (cover)
Highlighted by organic chemistry portal
5.One pot synthesis of useful heterocycles in medicinal chemistry using a cascade strategy
Guiyong Wu, Weiyu Yin, Hong C. Shen,* and Yong Huang*, Green. Chem. 2012, 14, 580-585. (cover)
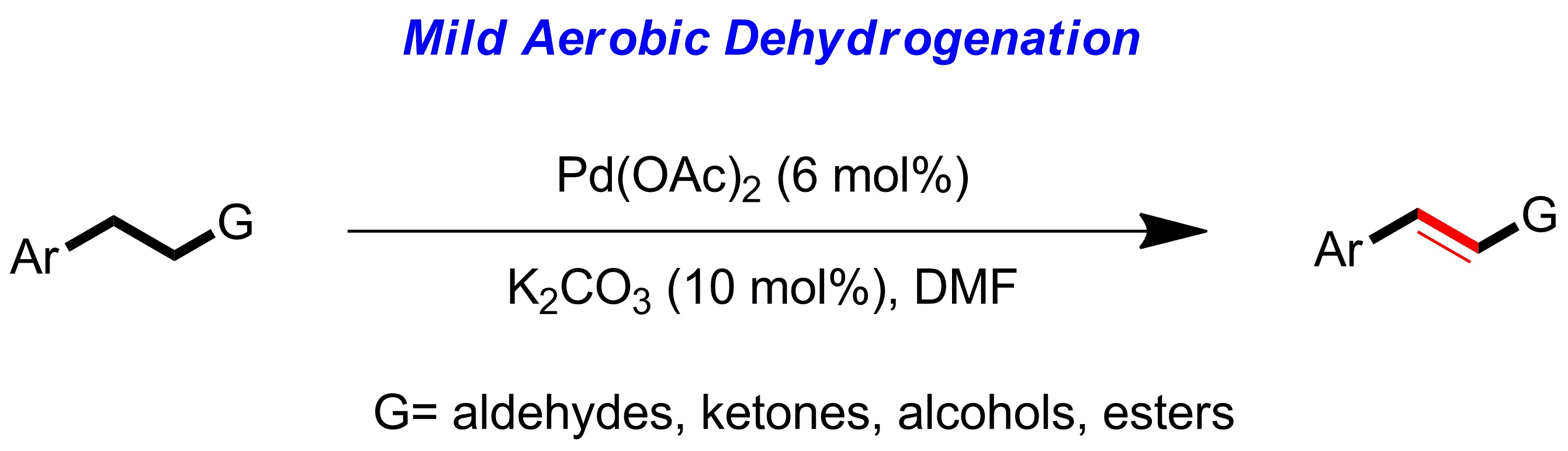
4.General palladium catalyzed aerobic dehydrogenation to generate double bonds
Weiming Gao, Zhiqi He, Yong Qian, Jing Zhao* and Yong Huang*, Chem. Sci. 2012, 3, 883-886.
Ying-Xiang Gao, Le Chang, Hang Shi, Bo Liang, Kittiya Wongkhan, Duangduan Chaiyaveij, Andrei.S. Batsanov, Todd.B. Marder,* Chuang Chuang Li,* Zhen Yang,* Yong Huang* Adv, Synth. Catal. 2010, 352, 1955-1966.
2. Enantioselective Organo-Cascade Catalysis
Yong Huang, Abbas M. Walji , Catharine H. Larsen, and David W. C. MacMillan *, J. Am. Chem. Soc. 2005, 127, 15051-15053.
1. Hydrogen bonding: Single enantiomers from a chiral-alcohol catalyst
Yong Huang, Aditya K. Unni, Avinash N. Thadani and Viresh H. Rawal*, Nature 2003, 424, 146-146.
Patents:
1. “A method to Prepare Nitriles” 201210447319.X
2. "Methods of Preparing Ketones and Their Derivatives" 201110252324.0
3. "Preparation of Cyclopropyl Compounds Containing Pyridine and Pyrimidine Rings as GPR119 Receptor Agonists for the Treatment of Type 2 Diabetes and Related Diseases" WO2009129036
4. "Preparation of Bipiperidinyl Compounds as GPR-119 Agonists for Treating and Preventing Diabetes" WO2008085316
5. "Acyl Bipiperidinyl Compounds as G-protein Coupled Receptor GPR-119 Agonists, Their Preparation, Compositions Containing Such Compounds and Methods of Treatment" WO2008076243
6. "Methods of Performing Cycloadditions, Reaction Mixtures, and Methods of
Performing Asymmetric Catalytic Reactions" US 7230125
C