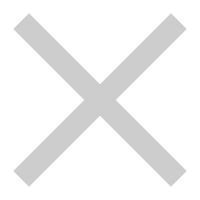
1. NHC “biomimeticproton-shuttle”catalysis
1.Chen, J.; Huang, Y.*, Asymmetric Catalysis with N-heterocyclic Carbenes as Non-covalent Chiral Templates. Nat. Commun. 2014, 5, 3437.
2.Yuan, P.; Chen, J.*; Zhao, J.*; Huang, Y.*, Enantioselective Hydroamidation of Enals by Trapping of a Transient Acyl Species. Angew. Chem. Int. Ed. 2018, 57, 8503.
2.Enamine catalysis with multi reactive sites

1.Wang,Z.; Li, X.; Huang, Y.*, Direct α-Vinylidenation of Aldehydes and Subsequent Cascade Gold and Amine Catalysts Work Synergistically. Angew. Chem. Int. Ed. 2013, 42, 14219.
2.Luo, C.; Wang, Z.; Huang, Y.*, Asymmetric Intramolecular α-Cyclopropanation of Aldehydes Using a Donor/Acceptor Carbene Mimetic. Nat. Commun. 2015, 6, 10041.
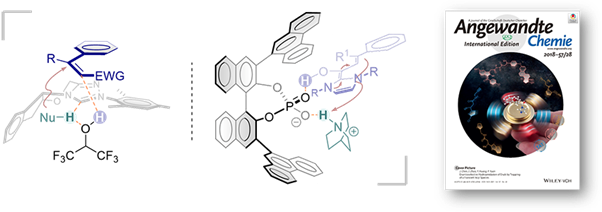
3.Light-induced proton transfer reaction
1.Wang, L.; Wu, F.; Chen, J.; Nicewicz, D. A.*; Huang, Y.*, Visible Light Mediated [4+2] Cycloaddition of Styrenes Synthesis of Tetralin Derivatives. Angew. Chem. Int. Ed. 2017, 56, 6896.
2.Wu, F.; Wang, L.; Chen, J.*; Nicewicz, D. A.*; Huang, Y.*, Direct Synthesis of Polysubstituted Aldehydes via Visible-Light-Catalysis. Angew. Chem. Int. Ed. 2018, 57, 2174.
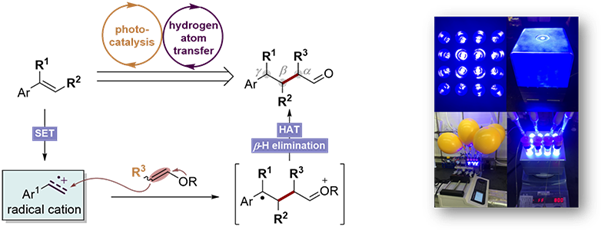
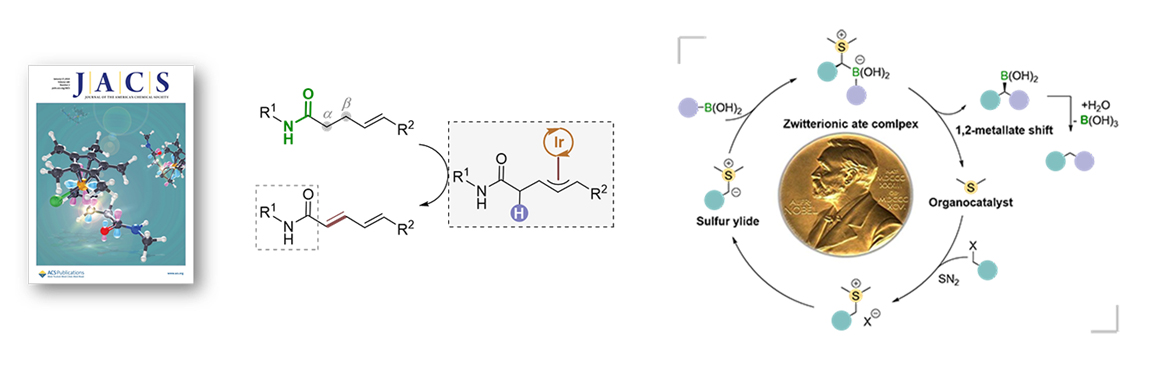
4.Catalytic functional group shift transformation
1.Wang, Z.; He, Z.; Zhang, L.; Huang, Y.*, Iridium-Catalyzed Aerobic α,β-Dehydrogenation of γ,δ-Unsaturated Amides and Acids: Activation of Both α- and β-C–H bonds through an Allyl–Iridium Intermediate. J. Am. Chem. Soc., 2018, 140, 735.
2.He, Z.; Song, F.; Sun, H.; Huang, Y.*, A Transition-Metal-Free Suzuki-Type Cross-Coupling Reaction of Benzyl Halides and Boronic Acids via 1,2-Metallate Shift. J. Am. Chem. Soc., 2018, 140, 2693.
5.Medical chemistry study of targeted antitumor drugs

1.Pan, P.; Chen, J. et al. Huang, Y.*; Hou, T.*, Structure-Based Drug Design and Identification of H2O-Soluble and Low Toxic HexacyclicCamptothecin Derivatives with Improved Efficacy in Cancer and Lethal Inflammation Models in Vivo. J. Med. Chem. 2018, 61, 8613.
2.Pan, P.; Yu, H. et al. Huang, Y.*; Hou, T.*, Combating Drug-Resistant Mutants of Anaplastic Lymphoma Kinase with Potent and Selective Type‑I 1/2 Inhibitors by Stabilizing Unique DFG-Shifted Loop Conformation. ACS Cent. Sci. 2017, 3, 1208.